All activities using genetically modified organisms (GMOs) and/or pathogens in the laboratory, animal house, greenhouse, hospital room or large-scale production installation are subject to authorisation by the regional authorities. The facilities concerned are mainly universities, scientific institutes, pharmaceutical companies and clinical diagnostics laboratories. A few companies carrying out microbiological tests as part of product quality control or environmental monitoring are also concerned.
In Belgium, the first contained use (CU) of genetically modified micro-organisms (GMMs) was notified in 1994 in context of recombinant enzyme production for medical or industrial use. In the same year there was also a contained use notified related to diagnostics and surveillance of zoonotic bacteria. In 1996 the first clinical trial of a medicinal product containing a GMM was notified. Since 1994, a total of 6661 contained use activities have been advised by the SBB. On average, 222 CU activities (and 105 dossiers) have been advised yearly (details of the yearly number of advices delivered since 2010 can be found in the graph below). The advices concern different types of notification: new activity (41% of the cases), changes in an existing activity (45% of the cases), renewal of an activity (14% of the cases) (activities are authorised for a limited period of time, e.g. 10 years for activities that do not evolve rapidly, such as diagnostics, and 5 years for activities that are expected to change rapidly, such as R&D).
It is important to note that the data provided on this webpage does not intended to represent the current situation of contained use in Belgium (i.e. the number of ongoing CU activities) but should be interpreted as an overview of what is advised annually by the SBB.
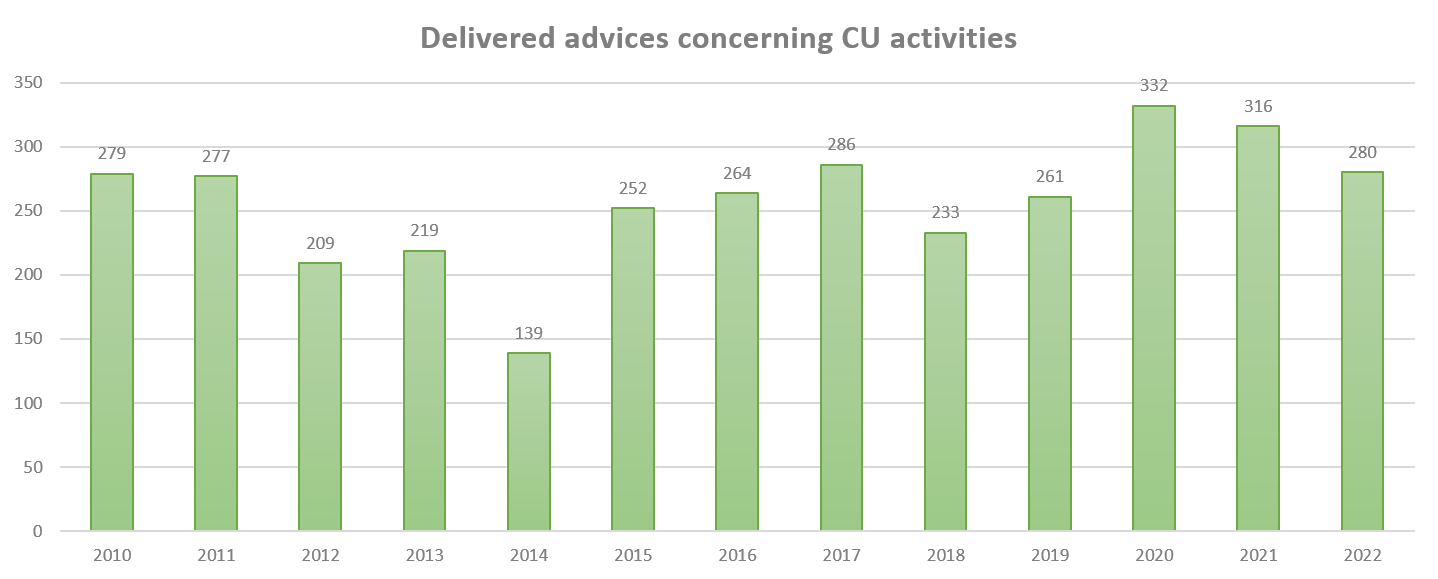
Figure 1: Number of advices delivered by the SBB on CU activities
An activity involving contained use of GMOs and/or pathogens is categorised from 1 to 4 according to an increasing scale of risk. Since 2013, 69% of the CU advices concerned risk level 2 activities, followed by risk level 1 (24%) and a minority of risk level 3 (7%), see graph 2. Until today no activities of risk level 4 are advised in Belgium. However, there are activities where GMMs of class of risk 4 are manipulated but which do not require a containment level 4.
The biological material is also categorised from 1 to 4 according to an increasing risk for human/animal health and the environment. Graph 3 shows average annual number of activities (since 2013) according to the highest risk class of biological materials for non-GMMs, GMMs and GMO manipulated per activity in the period 2013-2021. In total, 46% of the activities assessed by the SBB concerned exclusively GMOs or GMMs, while 30% of the activities concerned exclusively non-GM pathogenic organisms and 23% of the activities concerned both pathogenic organisms and GMOs/GMMs.
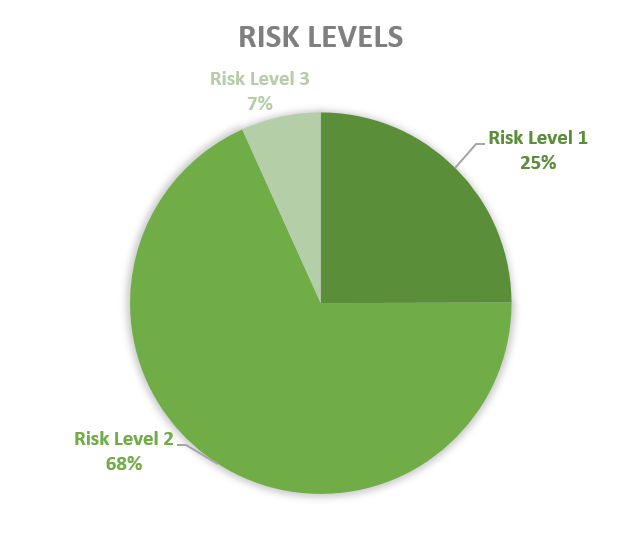
Figure 2: Risk levels of CU activities advised by the SBB (period 2013-2023)
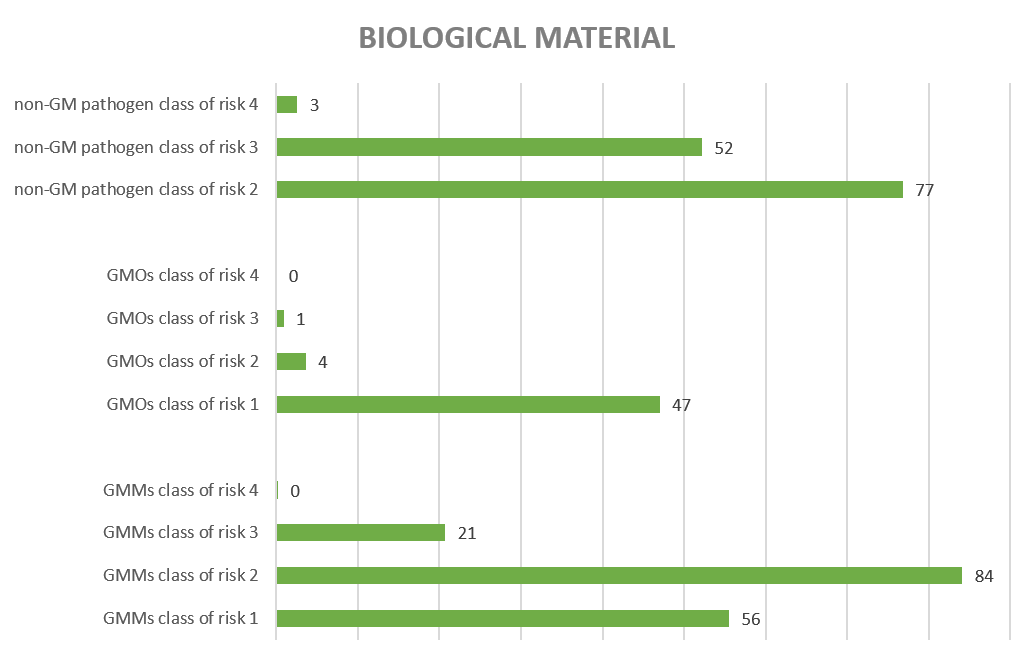
Figure 3: Average number of activities according to highest risk class of biological materials (type of non-GMMs, GMMs and/or GMO) manipulated in the activity (period 2013-2023)
Since 2013, most of the contained use activities in Belgium were performed in a lab facility (L) (73%), followed by animal facilities (A) (15%), hospital rooms (HR) (clinical trials, details see https://www.biosafety.be/content/clinical-trials-gmos-some-figures) (5%), plant facilities/greenhouses (G) (5%) and large scale production facilities (LS) (2%).
In Belgium, the number of exploitants operating at least one high-containment facility currently stands at 56, with 28 in Flanders, 20 in Wallonia and 8 in Brussels. This includes laboratories (L3 & L3-BSE), large-scale production facilities (LS3) and animal houses (A3). There are no level 4 facilities recorded in Belgium today.
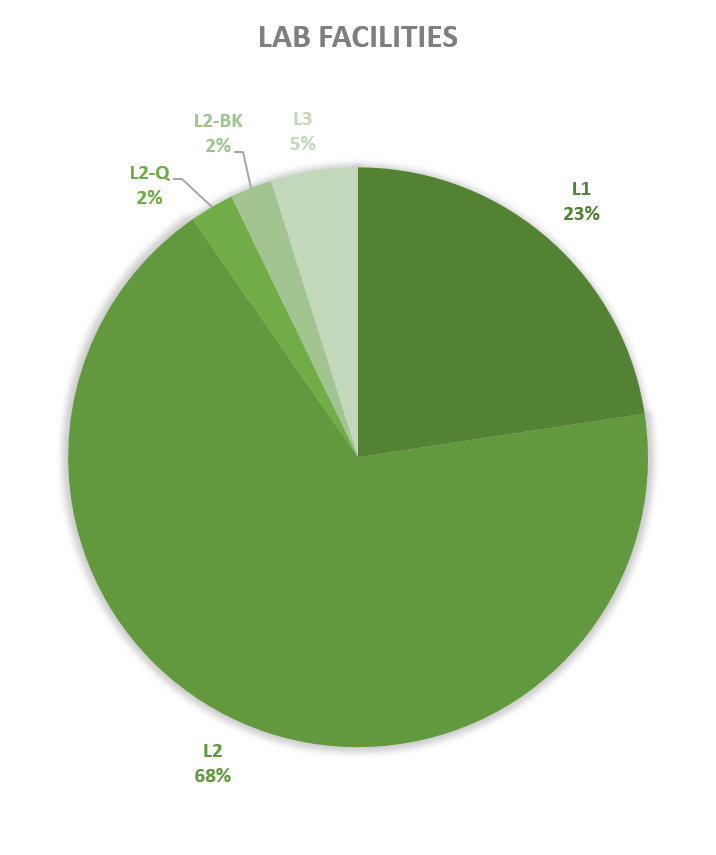
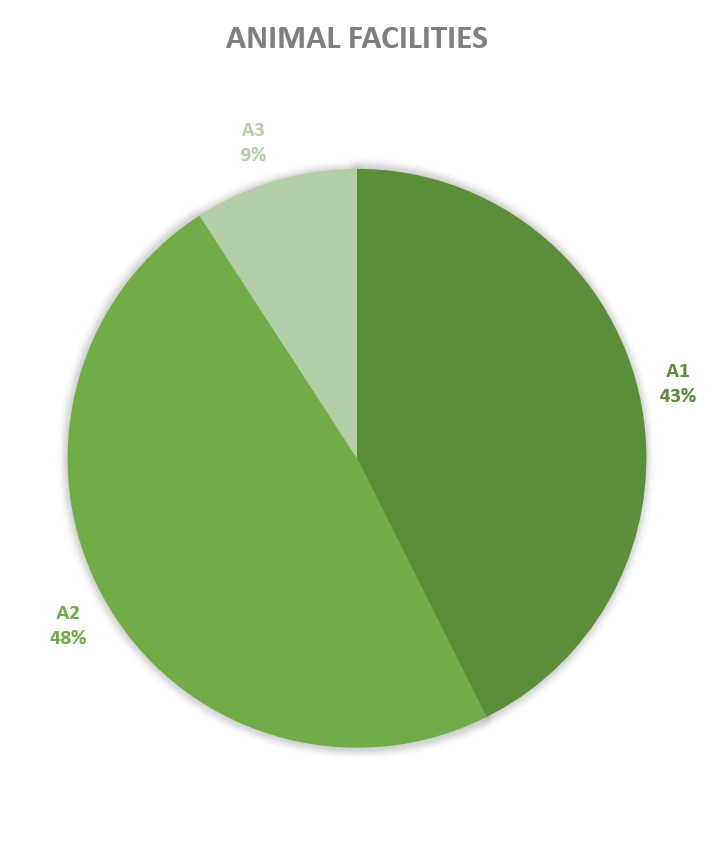
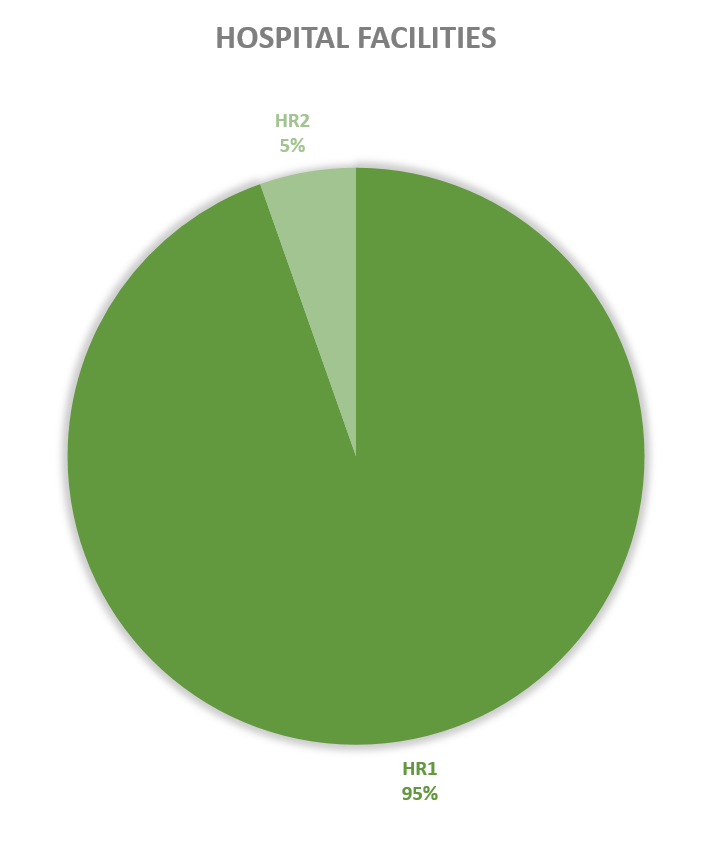
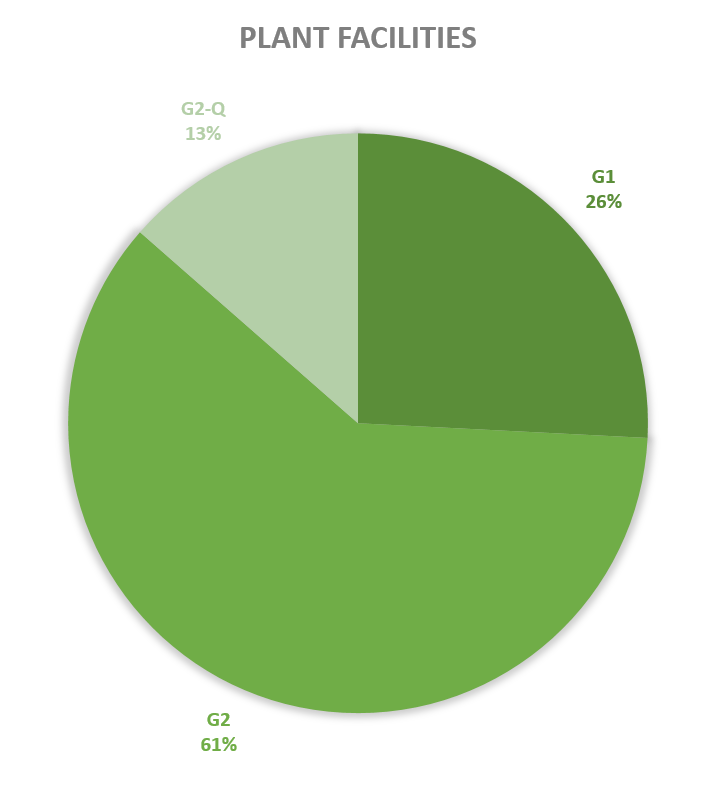
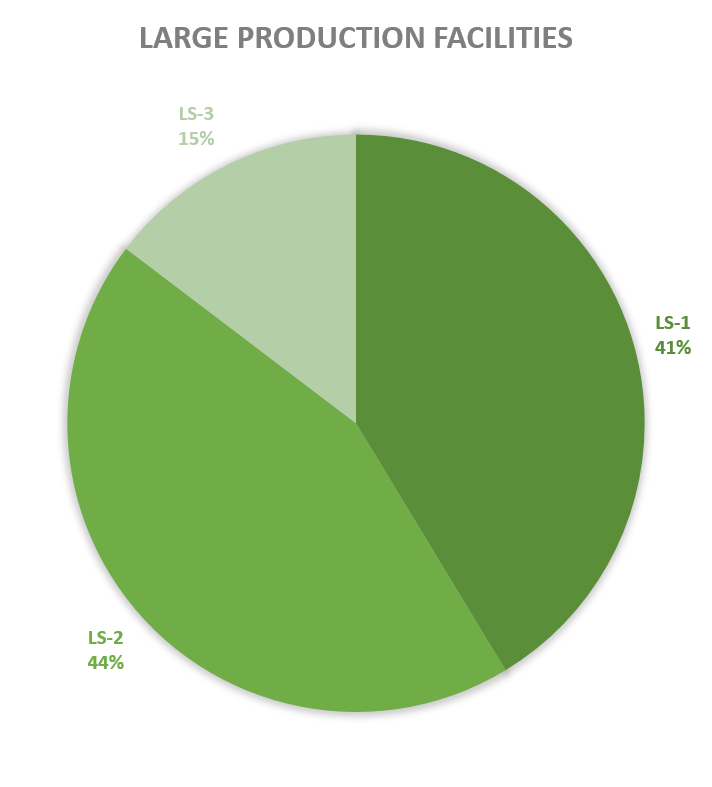
Figures 4-8: Overview (per year) of the ratio of containment levels adviced within the different types of CU installations in period 2013-2023. Add info (1) -Q = Specific containment requirements in the context of activities involving handling of quarantine organisms, (2) -BSE = Specific containment requirements in the context of handling of non-conventional agents and (3) -BK = Specific containment requirements in the context of primo-identification of pathogens of the type Mycobacterium complex, Brucella,... . Details of requirements, see here in Dutch / here in French
Activities of contained use notified in Belgium since 2018 concern mostly R&D activities (46% of the cases), followed by clinical trials (10%), diagnostics (10%), quality control (11%) and, to a lesser extent, storage (9%), production (5%), education (5%) and/or shipment (3%). CU activities are mainly located in universities and scientific institutes (75%), followed by private companies and/or laboratories (16%) and hospitals and/or clinics (9%).
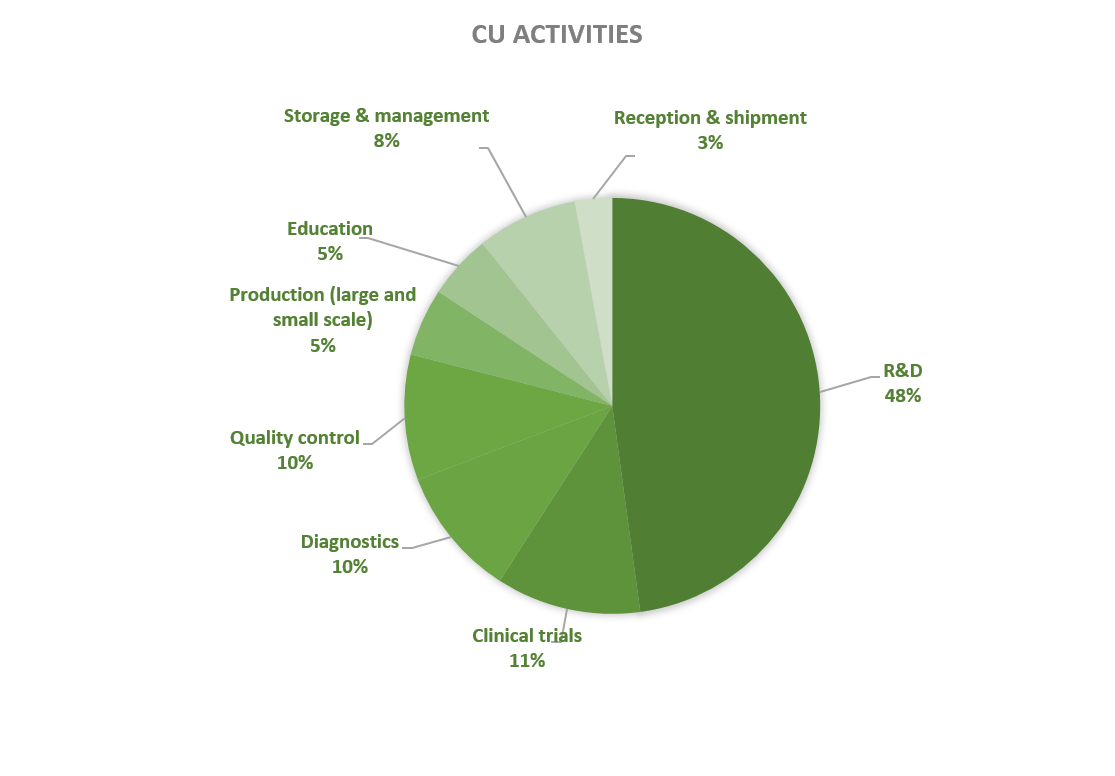
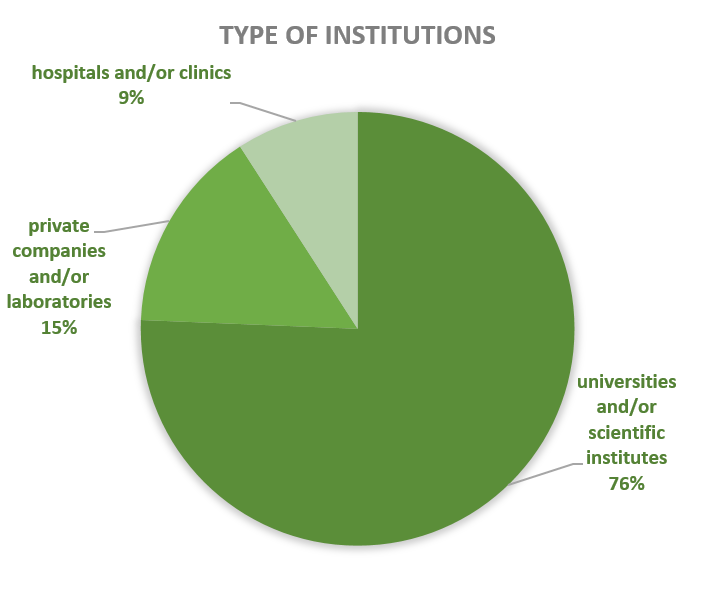
Figures 9-10: Types of CU activities and facilities advised by the SBB (period 2018-2023)
